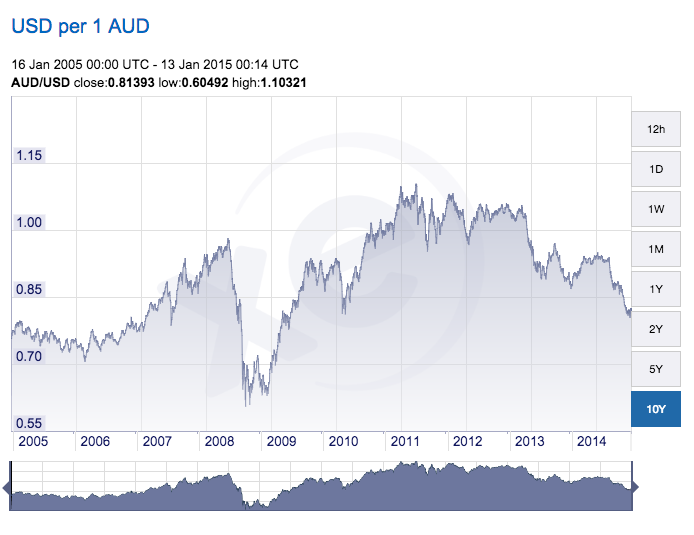
FX-rates reduce Australian clinical trial costs
The cost of having clinical trial work done in Australia has moved down dramatically recently and likely to stay down while the China demand for mined raw materials remains low and the Australian...