Experience, Expertise, Performance in Data Management Services in Clinical Trials
The technology you adopt for data capture and management drives the integrity and efficiency of your clinical study conduct.
Specialists in data management since 1987, our high level of attention to detail combined with our well developed procedures and common-sense approach across various clinical trials over multiple countries, have earned Datapharm Australia a strong reputation for providing excellence in all data management processes. As information management specialists, we can meet all your data management needs and are able to tailor our services to meet your budget.
Our data management specialists are highly experienced in handling medical and scientific data. All data is thoroughly reviewed by appropriately trained and experienced staff to ensure data integrity. All our data management processes are regulated according to closely controlled SOPs and in accordance with ICH GCP, FDA 21 CFR part 11 and CDISC/CDASH standards. These established data management systems can offer you significant time and cost savings.
Datapharm Australia team takes very seriously its obligation to protect the privacy, confidentiality and security of all information entrusted to us.
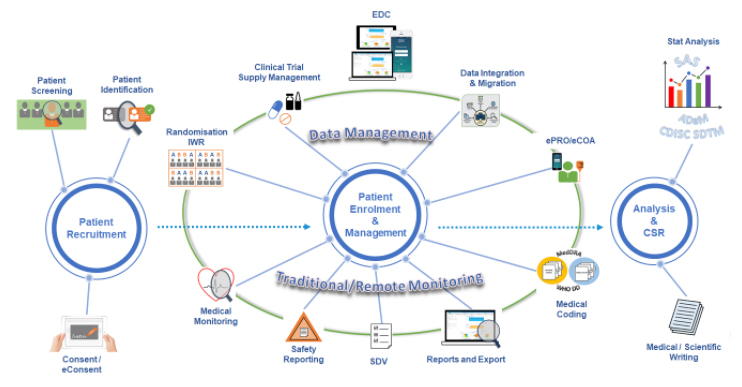
Datapharm Australia’s data capture and management solutions enable interoperability between collection database, electronic clinical outcome assessment (eCOA), eConsent, randomisation and trial supply management, etc. capabilities.
Datapharm Australia’s solutions eliminate complex, manual processes and deliver higher quality data for faster decision making and real-time inspection readiness. Our solutions drive critical reductions in study build time, query volume, data correction rates, and reporting turnaround time.